Many metals and metallic alloys, thanks to their properties and functions, are critical to nearly every technology and industry. However, metal resources are non-renewable and undergo some mass loss during their use. Aluminium, with its many superior properties, offers significant potential for improvement in process metallurgy, both through sustainable material design and its recyclability.
In general terms, process metallurgy underlies the extraction, refining and recycling of metals and is based on knowledge of transport phenomena, thermodynamics and reaction kinetics and their interactions in high-temperature heterogeneous metallurgical processes.
The often neglected concept of entropy is a key concept for both primary and secondary aluminium metallurgy.
Entropy
The word entropy comes from the ancient Greek word “entrope”, meaning “return”. Understanding the role of entropy in process metallurgy is challenging because the concept of entropy cannot be observed directly.
The most general and popular definition of entropy is associated with disorder.
The first law of thermodynamics, the Conservation of Energy, is a fundamental law. We can write this law as “the energy change of a system during a process is equal to the energy interaction of the system.” However, the same consideration does not apply to entropy. During a process, the entropy change of a system is greater than or equal to the entropy interaction of the system. This equality applies only to ideal processes. Therefore, in real processes, the entropy change of the system is greater than the entropy interaction of the system. The difference is always positive and equal to the entropy production.
The second law of thermodynamics, or the principle of increasing entropy, demonstrates that processes are irreversible; in other words, they are not (spontaneously) reversible. Life and all life-related processes are irreversible. For example, these include natural processes, such as the oxidation of metals, as well as technical processes, such as the combustion of fossil fuels in internal combustion engines or the production of metals from ores. The concept of entropy, introduced in thermodynamics to reflect this natural reality, allows for quantitative expressions about the efficiency of energetic and material transformations.
The importance of entropy is that it can quantify irreversibility.
The more concentrated the energy, that is, the lower its entropy, the more likely we are to do work with it. The more dispersed the energy, that is, the more increased its entropy, the less likely we are to do work. For example, we can’t generate energy from randomly scattered puddles of water on Earth’s surface. Still, we can do meaningful work by collecting water on one side of a dam and letting it flow in a controlled manner to the other side, thereby generating the electrical energy we need.
All human activity increases entropy, but many of the things we do are also helpful, improving our quality of life and making our work easier. By using the concept of entropy, we can make these activities less irreversible. Therefore, we can also define entropy as “meaningful disorder.”
Entropy production measures the irreversible loss of energy and the dissipation of matter, a key physical constraint on achieving global sustainability goals. In this context, recycling represents an opportunity.
Entropy and Recycling
Both natural and anthropogenic systems (e.g., industrial systems) essentially operate as entropy production mechanisms, converting primary natural resources (with low entropy) into waste (with high entropy). Humans recycle their waste to reduce the environmental impact of their systems. However, every human activity, including those aimed at recycling, causes global entropy to increase. According to this approach, entropy can be a measure of the environmental sustainability of anthropogenic systems, meaning that the greater the entropy produced by human activities, the greater their environmental impact.
Is aluminium scrap waste or a new raw material?
According to 2023 data from the International Aluminium Institute (IAI), the global average electrical energy consumed per tonne of primary aluminium in the electrolysis process alone was 13.2 kWh/kg Al. 67% of this energy came from fossil fuels, releasing 16.6 t CO2e per tonne of aluminium into the atmosphere. However, because aluminium metal behaves as an energy bank, the embodied energy stored in the primary production process allows recycling processes to consume between 5% to 8% of the primary production. The same relationship applies to CO2e emissions. Globally, the average CO2e emission from aluminium recycling processes is 0.6 t per tonne of aluminium according to the IAI and 0.5 t according to Hydro.
In other words, when we approach waste as a “new raw material,” the environmental damage will be more manageable because a lower amount of entropy will be produced.
The recycling process allows the production of secondary products with lower entropy as a result of mechanical and thermal processes, as scrap has become complex, worn out and disordered. In other words, recycling is an important tool in the challenge to reduce entropy.
Pre-consumer scrap with lower entropy
Post-consumer scrap with higher entropy
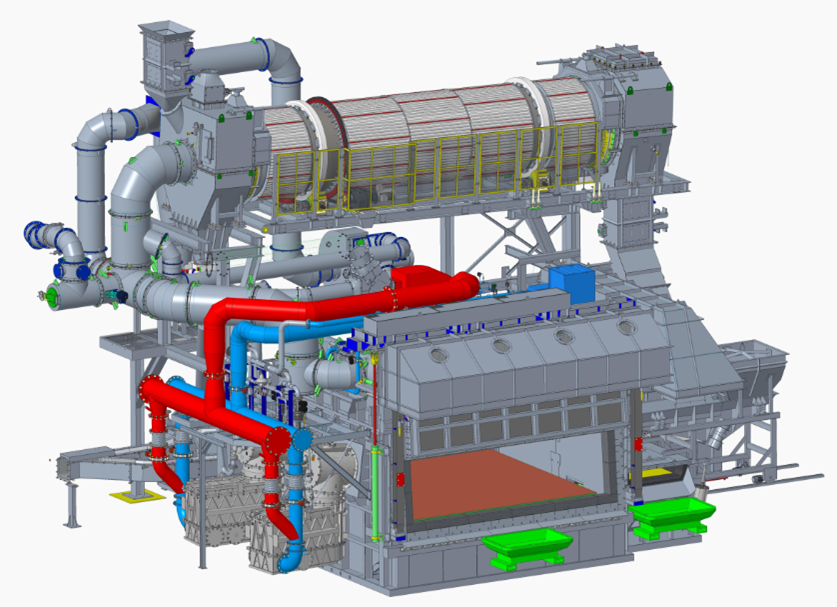
State-of-the-art Combined Decoating Melting system (CDM-Insertec)
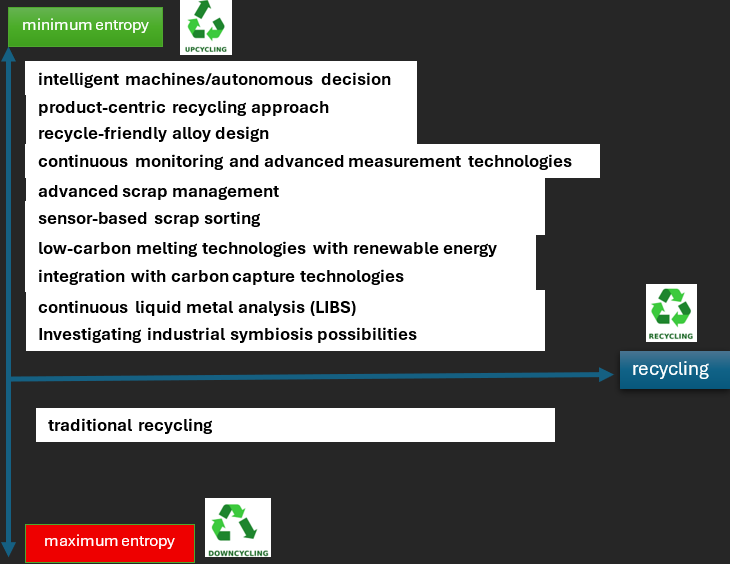
If a material is recycled into a lower-quality product, entropy increases (downcycling). The material loses some of its original order and value, making it difficult to return it to its original high-quality state. Consequently, more efficient recycling processes and less recycling can lead to lower entropy in the overall system, leading to reduced energy consumption, reduced waste and improved resource utilisation. Therefore, sustainable practices aim to minimise entropy as much as possible and maintain a closed material cycle (upcycling).
Conclusion
When you understand the concept of entropy, the increase in chaos within a system and the role technology plays in it, both climate change and resource depletion take on new meaning. Entropy is what unites everything we do to this planet: we consume all the low-entropy, high-value resources and convert them into high-entropy (well-diluted) pollution and waste heat.
Future materials management systems will prioritise reducing the entropy cost of recycling. With today’s technological advancements, we have many tools at our disposal to avoid downcycling and achieve upcycling. In other words, we can create a digital ecosystem for secondary aluminium production.
Even after recycling processes that attempt to reduce entropy, achieving original perfection is impossible because there is constant disorder in the system. In other words, it is impossible to meet all primary aluminium needs from secondary sources. However, every gram of primary aluminium substituted for secondary material will make a significant contribution to achieving sustainability goals.
That is, it will contribute to reaching meaningful disorder from disorder.
It’s important to remember that every law is a limit. Entropy is also an expression of limitation. However, limits also create new free spaces. In this sense, engineering is not just system design; it’s the opening up of a universe of new possibilities.
References
- Taner Derbentli, “On Thermodynamics”, https://web.itu.edu.tr/derbentlit/wterm5.htm
- Halvor Kvande, “Net-Zero Emissions from Primary Aluminium Production – Is This Technically and Economically Possible?, ICSOBA International Conference and Exhibition, November 2023, Dubai
- Dimitros Marinos-Kouris, Andreas Mourtsiadis, éEnvironmental Limits of Industrial Symbiosis: The Case of Aluminium Eco-Industrial Network, Fresenius Environmental Bulletin, Volume 22, No:12, 2013
- https://pollution.sustainability-directory.com/term/entropy-generation-in-sustainability/
- Stephon Alexander, “Yaşam, Entropi Değişimlerinin Kaçınılmaz Bir Sonucu Olarak Açıklanabilir mi?”, https://evrimagaci.org/yasam-entropi-degisimlerinin-kacinilmaz-bir-sonucu-olarak-aciklanabilir-mi-12361
- Çağrı Mert Bakırcı, Anıl Kocabaldır, “Termodinamik Yasaları ve Evrim: Entropi Nedir? Evrim, Termodinamiğin İkinci Yasası ile Çelişir mi?”, https://evrimagaci.org/termodinamik-yasalari-ve-evrim-entropi-nedir-evrim-termodinamigin-ikinci-yasasi-ile-celisir-mi-103